
03 June 2024
Murdoch Children's Research Institute (MCRI) and Incepta Pharmaceuticals, based in Bangladesh, have embarked on a collaboration aimed at bringing life-saving innovation to the global healthcare landscape.

MCRI has announced the signing of a non-exclusive licensing agreement with Incepta Pharmaceuticals, marking another milestone in its journey to reduce the impact of rotavirus, the most common cause of severe diarrhoea in infants and young children worldwide.
RV3-BB, adapted and developed from a naturally occurring human strain of rotavirus discovered in Melbourne by Professor Ruth Bishop AC and colleagues inthe early 1980’s , offers early protection against dehydrating diarrhoea from birth. The RV3-BB vaccine, intended for neonatal or infant dosing schedules as part of routine Expanded Program on Immunization (EPI) vaccinations, has the potential to save hundreds of thousands of children’s lives annually.
In Bangladesh alone, where more than two million babies are born every year, the impact of RV3-BB could be profound. Globally, rotavirus claims the lives of about 450,000 children under the age of five annually, making the need for accessible and effective vaccines urgent.
MCRI Professor Andrew Steer, Director of Infection, Immunity and Global Health said, “This collaboration represents a significant step forward in our mission to protect vulnerable children from the effects of rotavirus infection. By joining forces with Incepta Pharmaceuticals, we are poised to make RV3-BB readily available to communities in need worldwide.
“MCRI's relentless pursuit of accessible and scalable vaccine solutions underscores our commitment to global health equity.”
Abdul Muktadir, Chairman & Managing Director of Incepta Pharmaceuticals said, "We are proud to partner with MCRI in this groundbreaking endeavour. Together, we are committed to leveraging our expertise and resources to ensure that rotavirus vaccine (RV3-BB) reaches the millions of childrenwho need it most, safeguarding the health of future generations."

Abdul Muktadir, Chairman & Managing Director, Incepta Pharmaceuticals Ltd.
With Incepta Pharmaceuticals' established reputation as a leading pharmaceutical company in Bangladesh, equipped with the capacity to produce vaccines at scale, RV3-BB vaccine is poised to make a transformative impact on childhood health worldwide.
Media Contact:
Incepta Pharmaceuticals ltd.
Email: info@inceptapharma.com
About Murdoch Children’s Research Institute:
Murdoch Children's Research Institute is the largest child health research institute in Australia committed to making discoveries and developing treatments to improve child and adolescent health in Australia and around the world. They are pioneering new treatments, trialling better vaccines and improving ways of diagnosing and helping sick babies, children and adolescents. It is one of the only research institutes in Australia to offer genetic testing to find answers for families of children with previously undiagnosed conditions.
About Incepta Pharmaceuticals:
Incepta Pharmaceuticals Ltd is a leading pharmaceutical company based in Bangladesh, specializing in the development, manufacturing, and marketing of a wide range of pharmaceutical products since 1999. With a strong commitment to healthcare improvement, Incepta has been at the forefront of producing innovative vaccines and medications that meet the highest standards of quality and efficacy.

18 Jan 2023
Imperial College London, the world's leading medical education and research institute, has expressed interest in working with Incepta Pharmaceuticals, a renowned vaccine manufacturing of the country. Professor Robin Shattock, head of the research team at the Department of Infectious Diseases, Imperial College, London, visited Vaccine and Injectable plants of Incepta at Savar and Dhamrai . He expressed his great appreciation and said that Incepta’s management is very commendable. Mr. Abdul Muktadir, Chairman and Managing Director of Incepta Pharmaceuticals Ltd., was present during the on-site inspection of vaccine pants. At the end of the visit, mRNA vaccine expert Professor Robin John Shattock reiterated to support Incepta in technology and research to further develop its ability towards a variety of life-saving new vaccines.
Mr Abdul Muktadir, chairman and managing director of Incepta Pharmaceuticals Ltd. stated that Professor Robin John Shattock of Imperial College has helped Incepta to develop protein subunit vaccine in the past. In this continuation, he will also provide technical assistance in the development of other vaccines.
He also added that Incepta is now involved in the development of therapeutic vaccines. We will now jointly research in both replicating and non-replicating mRNA vaccines. We will also participate in future research on mRNA and therapeutic vaccines. He said, Professor Robin is a world-famous scientist. There are many scientists of this country who will be trained with his help and new areas of research will be opened. We have already trained two scientists of Incepta from Imperial College. Many more will be trained soon. This will also open the door to manufacture basic materials (seeds) of vaccine in Bangladesh. We believe that a new era has dawned in the field of research on vaccines and drugs. By doing this it will be possible to bring better benefits to the people.

Jan 09, 2023
Sarah Gilbert, a vaccinologist and co-inventor of AstraZeneca coronavirus vaccine, visited the office of Incepta Pharmaceuticals today.
Gilbert is a professor of vaccine science at the Jenner Institute, Oxford and Nuffield Department of Clinical Medicine of the University of Oxford. She led the development of the Oxford-AstraZeneca vaccine for Covid-19.
She was awarded the prestigious Albert Medal by the Queen in 2021 and Damehood by the Princess Royal at Windsor Castle in 2022 for her contribution to developing the Oxford-AstraZeneca vaccine.
Abdul Muktadir, chairman and managing director of Incepta Pharmaceuticals, and Hasneen Muktadir, vice-chairman, welcomed Gilbert at the Incepta head office in Tejgaon, Dhaka.
Future challenges of vaccine production in Bangladesh, availability of vaccines to common people, and capacity of vaccine production were discussed during her visit. Various aspects of modern and advanced technologies in vaccine production were deliberated and Gilbert gave her guidance on how Incepta can incorporate these modern technologies, said the drug maker.

2 August 2022
A seminar titled 'An Effort to Build a Cervical Cancer Free Bangladesh' and the unveiling of Incepta's new vaccine Papillovax were held at the capital's Pan Pacific Sonargaon Hotel on Tuesday, August 2.
The seminar discussed the need for HPV vaccine (human papillomavirus) in the country and how regular screening and introduction of HPV vaccine (human papillomavirus) Papillovax can contribute to achieving a cervical cancer free country.
Dr. expert doctor in the seminar. TA Chowdhury and National Professor Shahla Khatun delivered speeches as chief guests. The seminar was presided over by the president of OGSB (Obstetrical and Gynecological Society of Bangladesh) Professor Dr. Ferdowsi Begum. Professor Dr. The seminar started with welcome address by Gulshan Ara.
Former Chairman of Gynecology Oncology Department of Bangabandhu Sheikh Mujib Medical University Prof. Dr. Sabera Khatun and Manager (MSD) of Incepta Pharmaceuticals Farhana Laiju presented on the subject in the seminar. Scientific Secretary of OGSB, Professor Sheikh Jinnat Ara Nasreen moderated the excellent question and answer session.
Incepta Pharmaceuticals Ltd. expressed thanks on behalf of Incepta at the seminar. Executive Director Dr. EH Arefin Ahmed. Finally, Incepta officially unveiled PapilloVax, heralding a new era in cervical cancer prevention.
To eliminate cervical cancer, the HPV vaccine is the first step. There was a huge gap in this vaccine in Bangladesh for the past few years. Incepta Vaccine Ltd., the country's first vaccine manufacturing company, has come forward to fill this gap. and started marketing the country's first cervical cancer vaccine, Papillovax. It is a matter of pride for the country, which will pave the way to eradicate cervical cancer from the country.

18 July 2022
Incepta Vaccine Limited launched its cervical cancer vaccine "Papilovax" for the first time in Bangladesh as a local company.
Papilovax protects women against cervical cancer by countering the HPV virus responsible for the disease.
Women 9-45 years in age should receive three doses of Papilovax. The second dose should be taken one month after the first dose and the third dose should be received six months after that.
Completing the full course of this vaccine will reduce the risk of developing cervical cancer throughout a woman's lifetime.
Cervical cancer is the second deadliest among different types of cancers that cause female deaths in Bangladesh. Every year more than 10,000 women die of cervical cancer in the country and more than five crore women are at risk.
Papilovax is marketed in modern pre-filled syringes. The full dose in pre-filled syringes is manufactured in an aseptic environment and marketed at controlled temperatures in fully sterile packaging.

Today 16th August, Monday at 3.00 pm an agreement has been signed between the Sinopharm of China, Ministry of Health and Family Welfare, People’s Republic of Bangladesh and Incepta Vaccine Ltd, renowned vaccine manufacturing company of the country, at the auditorium of Bangladesh College of Physicians and Surgeons (BCPS), Mohakhali Dhaka in order to produce the Sinopharm covid vaccine in Bangladesh.
Honorable Minister of Health and Family Welfare Mr. Zahid Maleque MP was the Chief Guest at the signing ceremony. Honorable Foreign Minister Dr. A. K. Abdul Momen MP was present as the guest of honor.
Mr. Masud Bin Momen, Senior Secretary, Ministry of Foreign Affairs, His Excellency, People’s Republic of China Li Jiming, Major General Md, Mahbubur Rahman, Director General, Directorate General of Drug Administration (DGDA), and Mr Abdul Muktadir, Chairman of Incepta Vaccine Ltd and Incepta Pharmaceuticals Ltd were also present in the program as Special guests. Prof. Dr. Abul Bashar Mohammad Khurshid Alam, Director General, Directorate General of Health Services (DGHS) gave a welcome speech. Mr. Lokman Hossain Miah, Senior Secretary, Health Service Division, Ministry of Health and Family Welfare presided over the function.
In addition, Counselor of the Department of Asia Affairs of the Ministry of Foreign Affairs of China Ji Rong and the Chairman of Sinopharm Mr Liu Jingzhen were connected online from Beijing and delivered speech as special guests. Mr. Fu Qiang, Chief Engineer of Sinopharm gave the welcome speech on behalf of Sinopharm.
Aiming to co-produce the China's Sinopharm vaccine in Bangladesh, the Memorandum of Understanding was signed by Honorable Minister Mr. Zahid Malek MP, His Excellency, People’s Republic of China Mr. Li Jiming and Abdul Muktadir, Chairman of Incepta Vaccine Ltd and Incepta Pharmaceuticals Ltd on behalf of Ministry of Health and Family Welfare, Sinopharm and Incepta Vaccine Ltd respectively.
Speakers at the event said that a new direction has been unveiled with this agreement in the production of covid vaccine in Bangladesh in this Mourning August. It was expected that through this agreement, Incepta Vaccine Ltd, would produce the Sinopharm Vaccine very soon.


For world cup football 2018, Incepta Vaccine Ltd sponsored a prediction game in the daily newspaper Prothom Alo which continued throughout the month. During this event, two winners had been selected in every week and finally total eight winners have been selected.
In the last 5th August, 2018 an award ceremony had been arranged with the all winners in the premises of the head office of Incepta Pharmaceuticals Ltd. All winners had been appreciated with attractive gifts. During this time, all participants gave their gratitude to the Prothom Alo and Incepta. The ceremony had packed up with the photo session. The COO of Prothom Alo and the General Manager of Incepta Pharmaceuticals were present in the program.

The day was observed with colorful rally and different awareness programs
Like every year, Incepta Vaccine Ltd. observed World Hepatitis Day 2018 on July 28th in Bangladesh with the collaboration of National Liver foundation of Bangladesh.
To observe the day, National Liver Foundation of Bangladesh (NLFB) conducted different awareness-building events where the campaign was “Find the Missing Millions”. The theme of the day was “Eliminate Hepatitis” by 2030’.
The colorful rally includes Roller skates, Horse carts and Motorbike started from Manik Mia Ave, crosses the main road of Dhaka city and ended at Daily star Auditorium, Kawranbazar. Doctors, Patients, members of Youth Club of Bangladesh and many University and college students actively participated in the rally and other events of the day. Moreover, A seminar was held at the Daily star Auditorium. National Professor Jamilur Raja Choudhury, Chairman of National Liver Foundation of Bangladesh, chaired the seminar. Ekushey Padak winner Prof. MirzaMazharul Islam, renowned surgeon & member Board of Adviser of the foundation was the chief guest. Prof. Mohammad Ali, Secretary General, NLFB delivered the keynote speech. He highlighted the various ongoing activities of the foundation and awareness events especially NOhep network and Find the Missing Million campaign in Bangladesh.




Incepta Vaccine Ltd. observed World Rabies Day 2017 on September 28 in Bangladesh. The day was observed with full of events e.g. rally, discussion on rabies and its preventive measures in different institutes with presence of doctors, nurses and other healthcare professionals.
"Rabies: Zero by 2030" with this theme the rally was started from Infectious Disease Hospital (IDH), Dhaka and ended at Krishibid Institute at Farmgate, Dhaka with the participation of Dr. Syad Ahsan Touhid, Sr. Consultant (Acting), IDH.
Besides, the day was also observed at Mohammad Ali Hospital, Bogra; Abdul Malek Ukil Medical College, Noakhali etc. sponsored by Incepta Vaccine Ltd. The MPOs, Area Managers and Regional Managers of Incepta dressed with the T-Shirt designed with logo of rabies day and Incepta.


ELIMINATE HEPATITIS, NOhep Drive 2017
Incepta vaccine ltd observed this day with the collaboration of the National Liver Foundation of Bangladesh by conducting different events throughout the country to spread the knowledge of Hepatitis B and eliminate it. This year the day was observed with the theme of ELIMINATE HEPATITIS,NOhep Drive.
A nationwide viral hepatitis awareness campaign throughout Bangladesh named as ELIMINATE HEPATITIS Nohep Drive 2017 was launched by the National Liver Foundation of Bangladesh (NLFB) on July 10, 2017, on the eve of World Hepatitis Day 2017 with the active participation of Incepta. This is in the light of the global viral hepatitis movement “NOhep” of World Hepatitis Alliance.
This event was started at The Central ShaheedMinar (Martyr’s Monument) at capital city Dhaka. NOhep branded Van started to move from Dhaka carrying volunteers, leaflets, banners, audiovisual presentations, viral hepatitis awareness questionnaires, posters, and other NOhep merchandise.
This Nohep van traveled all the EIGHT DIVISIONAL HEADQUARTERS of Bangladesh, starting from Dhaka>Rongpur>Rajshahi>Mymensingh>Sylhet>Chittagong>Barisal and finally to Khulna.
These 12 days long tour traveled more than 2000 kilometers of city and rural areas of the country. Conveyed messages to people of different walks of life, cultures and social status, with the aim of reaching the grass-root level. Distributed more than 30,000 leaflets, It also transmits messages to educational institutes, religious places, village markets, and public gatherings.
The program designed to touch most of the parts of Bangladesh to disseminate the message of NOhep campaign. It will act as groundwork for the elimination of viral hepatitis in Bangladesh.

Incepta Vaccine Ltd has launched its vaccine bulk manufacturing facility in Savar, outside the capital. Honorable health Minister Mohammed Nasim, MP inaugurated the facility on Dewan Idris Road in Zirabo on Thrusday, 28 July.
An internationally bulk manufacturing facility has been developed providing the best environmental conditions for performing the operations. The facility can manufacture both Bacterial and Viral bulk antigen. These make us less dependent on import and help to become a self-sufficient vaccine manufacturing country.
Addressing the inaugural ceremony, the minister said now Bangladesh is able to export medicines to 90 countries because of their high quality. Bangladesh is now capable of manufacturing life saving vaccine and successfully reduces the dependency on imported vaccines. ‘We will take the initiatives to get the WHO authorization which is needed for export of vaccines.’ Nasim said.
Director General of Drug Administration, Major General Md Mustafizur Rahman, Secretary General of Bangladesh Association of Pharmaceutical Industries, SM Shafiuzzaman were also present there as special guest.
Public-private collaboration among Incepta Vaccine Ltd, IVI, and icddr. Following technology transfer from IVI to Incepta Vaccine Ltd, clinical trials to start in Dhaka, Bangladesh
The International Vaccine Institute (IVI), International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), and Incepta Vaccine Ltd. announced today initiation of a clinical trial for Cholvax®, an inactivated oral cholera vaccine, in Dhaka, Bangladesh.The three groups are collaborating on its clinical development in order to register the vaccine in Bangladesh, the first oral cholera vaccine to be registered in the country.
The clinical trial will assess vaccine safety and immunogenicity in 2,052 people 1-45 years of age in Dhaka. As the trial sponsor, IVI will lead this research effort in collaboration with icddr,b, an international health research organization based in Bangladesh, and will also monitor the trial for quality assurance and provide technical support in data management and evaluation of the participants safety as well as immune response to the vaccine. Incepta, the first human vaccine manufacturer in Bangladesh, will be responsible for manufacturing and quality control of clinical lots of Cholvax® for the trial. The trial is supported by the Bill & Melinda Gates Foundation.
The partnership between IVI and Incepta began in July 2014 when IVI transferred the technology for oral cholera vaccine production and quality control methods to the company. Both IVI and Incepta are committed to developing the vaccine for the public sector in Bangladesh where a high burden of cholera exists. This is the first collaboration between IVI and Incepta. The two organizations are also working together on the development of a typhoid conjugate vaccine.
The vaccine is a bivalent killed whole-cell oral cholera vaccine given orally in two doses over a 14-day interval and intended for use in people one year old and above. It was originally reformulated by IVI and developed through an international public-private partnership led by IVI, with partners from Sweden, India, Vietnam, and South Korea, and with support from the governments of South Korea and Sweden, and the Bill & Melinda Gates Foundation.
“The collaboration with Incepta and icddr,b will result in a vaccine that can reduce needless suffering of so many people from a debilitating and potentially fatal disease,” said Dr. Jerome Kim, IVI’s Director General, “This collaboration is critical to achieving our goal of making vaccines available and accessible for the world’s most vulnerable and impoverished people.”


Incepta Vaccine Ltd. observed World Hepatitis Day 2015 on July 28th in Bangladesh. The day was observed with full of events in relation to create awareness to prevent viral hepatitis.
“Prevent Hepatitis, It’s up to you” with this slogan, a Cycle Rally has been initiated from the Central Shahid Minar and circled major roads of Dhaka city. Mr.Mahbubey Alam, Attorney General, Government of the People’s Republic of Bangladesh, Mr. Sayeed Khokon, Honorable Mayor, Dhaka City (South) and Prof. Mohammad Ali, Secretary General, National Liver Foundation of Bangladesh inaugurated the rally and wished to provide all out support for awareness activities for viral hepatitis.
Special program was broadcasted in television & different topics were transmitted in popular FM Radio whole day. The importance of prevention of viral hepatitis was also published in social media and in the newspaper. Posters, banners, leaflets were widely circulated on this auspicious day.
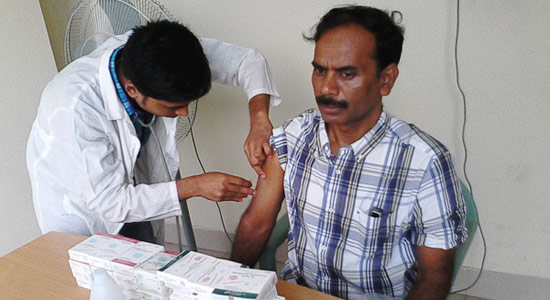
Vaccination for influenza and meningitis are two mandatory prerequisite for all Bangladeshi hajj pilgrims. Government of Bangladesh ensured vaccination of these two vaccines one month prior to the departure for Saudi Arabia.
In this year 2015, Incepta Vaccine Ltd is very much delighted to provide supply of influenza and meningitis vaccine to Government of Bangladesh for the hajj pilgrimages. Incepta Vaccine Ltd manufactured Influenza vaccine by the name of “Influvax” and Meningococcal polysaccharide vaccine by the name of “Ingovax” for meningitis. It has been a pride for the Incepta to be a part of this novel activity. Incepta pray for the safety and wellbeing of all Bangladeshi hajj pilgrim
Antivenom injection is the preparation of snake venom antiserum. In Bangladesh, about 8000 snake bites occur annually with more than 20% mortality. Snake bite causes serious systemic or neurological complications such as bleeding disorder leading to fatal hemorrhage, permanent disability or death. Treatment of snake bite is neglected and is left to traditional ozha or kaviraj which lead to death. Snake venom antiserum is the mainstay of treatment after snake bite. But in Bangladesh, this life saving antiserum was not available everywhere in every season due to there is no local manufacturer, lack of cold chain system. Now Incepta Vaccine Ltd has introduced snake venom antiserum for the first time in Bangladesh with the brand name of Antivenom. Antivenom is manufactured by applying high tech lyophilization technique so that it can be stored at room temperature and highly stable. Antivenom is available in 10 ml in vial after reconstitution that should be given as infusion.
Influenza vaccine protects against influenza viruses during influenza season. Flu virus causes respiratory infection which passes through the air and enters the body through nose or mouth. It spreads around the world in a yearly outbreak resulting in about 3 to 5 million cases. Flu leads to severe complication for chronically ill patient like Asthma, COPD, Diabetes, Heart disease. Flu can easily be prevented by vaccination before starting of every flu season. In Bangladesh, this life saving vaccine was imported, which was not available everywhere in every season. Now Incepta Vaccine Ltd has introduced Influenza vaccine for the first time in Bangladesh with the brand name of Influvax. Influvax vaccine is manufactured with the WHO recommended flu viruses (A/California/7/2009 (H1N1), A/Texas/50/2012 (H3N2), B/Massachusetts/2/2012 ) for northern hemisphere that are recommended for our country. Influvax is available in 0.5 ml vial that can be given intramuscularly.
Anti rabies vaccine is recommended as soon as possible after exposure to rabid animal to prevent rabies. However, in severe cases like WHO category-III bite only anti rabies vaccine is not enough to save life. In this case rabies immunoglobulin is recommended by WHO to ensure immediate protection. In Bangladesh, this life saving rabies immunoglobulin was imported, which was expensive and not available everywhere. Now Incepta Vaccine Ltd, a sister concern of Incepta Pharmaceuticals Ltd has introduced Rabies Immunoglobulin first time in Bangladesh with the brand name of Rabix-IG. Rabix-IG is available in 5 ml vial that can be given intramuscularly.
Tetanus antitoxin provides immediate protection against tetanus in tetanus prone wounds that can be happened in our life any time. About 73% cases of tetanus infection are caused by acute injuries or wounds. Bangladesh was fully dependent on imported tetanus antitoxin to prevent this deadly disease. Now Incepta Vaccine Ltd, a sister concern of Incepta Pharmaceuticals Ltd has introduced Tetanus antitoxin first time in Bangladesh with the brand name of Vaxitet-IG. Vaxitet-IG is available in 1 ml vial that can be given intramuscularly or subcutaneously.
Hepatitis B virus infection is one of the major burdens for Bangladeshi people. More than 10 million people are suffering from hepatitis B virus infection. Moreover, Bangladesh was fully dependent on imported vaccine to prevent this deadly infectious disease. Now Incepta Vaccine Ltd, a sister concern of Incepta Pharmaceuticals Ltd has introduced Hepatitis B vaccine first time in Bangladesh with the brand name of Hepa-B. Hepa-B is available in vial of 0.5 ml for pediatric and 1 ml for adult that can be given intramuscularly.
The first ever Human Vaccine manufacturing plant of Incepta Vaccine Ltd was officially inaugurated by the Honorable Health & Family Welfare Minister Prof. Dr. A. F. M. Ruhal Haque MP. Honorable Senior Secretary of Health & Family Welfare Ministry Md. Humayun Kabir, National Professor M. R. Khan, Director General of Drug Administration Major General Md. Abul Kalam Azad, Additional Director General of Directorate General of Health Services Prof. Dr. A.F.M. Saiful Islam and Director of Primary Health Care and Line Director of MNC & AH Dr. Syed Abu Jafar Md. Musa were present in the inauguration program as special guests. Abdul Muktadir, Managing Director of Incepta Vaccine Ltd, Mrs. Hasneen Muktadir, Director, Planning & Commercial, Director Jahiruddin Mahmud, Abdul Mukit Majumdar, Abdur Rashid Majumdar, Mahbubul Karim, Director, Technical Operations and other senior officials of Incepta were also present in the program.
In his speech, honorable Minister of Health & Family Welfare Prof. Dr. A. F. M. Ruhal Haque said “I am appreciating such initiative of Incepta Vaccine Ltd. At present our Government needs to spend several hundred crore Taka to import the necessary vaccines for EPI program. But, now we can save huge amount of foreign currency for such initiative of Incepta Vaccine Ltd.” Honorable Minister also visited the newly constructed 3 stored vaccine facilities which consist of 75000 square-feet, animal house which is required to ensure the efficacy of manufactured vaccines and also cold chain system which is important to maintain the efficacy of vaccine. He appreciated much to see such a world class vaccine plant and its facilities.
National Professor M. R. Khan said “Incepta has been supplying medicine of supreme quality from its inception. Their initiative makes a remarkable appreciation to reduce the dependency on foreign medicine.”
Abdul Muktadir, Managing Director of Incepta Vaccine Ltd said “in the near past we had to import essential vaccines from foreign countries. But now we are manufacturing vaccines in our own country. We have constructed a totally new and enormous vaccine plant according to the guidelines of WHO GMP.” He also said “the annual production capacity of our vaccine plant is about 18 crore vials which will not only fulfill the annual demand of EPI program but also provide us the opportunity to export these vaccines in the foreign countries.”
In conclusion, Mr. Muktadir promised to make all these high-tech and life saving vaccines available and affordable for the people of our country and sought overall assistance from the Government of Bangladesh for such endeavor.
Incepta Vaccine Ltd is the first Human vaccine company in Bangladesh, developed with primary objective to provide preventive medicine to vast majority of population at an affordable price. It is a new state of the art facility to manufacture vaccine in Bangladesh for the first time. The facility is fully compliant with WHO GMP guidelines. It has the following unique features:
Incepta Vaccine Ltd can produce any dose size from 0.1ml to 15 ml, with 4 different products simultaneously and has capacity to fill more than 2,40,000 ampoules and 3,60,000 vials per day. The yearly capacity is 180 million vials and ampoules. To conduct the immunization program of EPI, yearly demand of vaccine is 21 million vials and other than EPI yearly demand is 10 million vials which is increasing day by day. The production capacity of this facility is enough to meet the local demand and export.
Incepta Vaccine Ltd is now manufacturing and marketing Typhoid vaccine (Vaxphoid), Rabies vaccine (Rabix-vc) and Tetanus vaccine (Vexitet). In near future Hepatitis B, Polio, Measles, Rubella, Tetanus antitoxin, Rabies immunoglobulin, Pentavalent and all other necessary vaccines will be manufactured. Incepta Vaccine Ltd is committed to provide all high tech life saving vaccines to the people at affordable price.
Incepta Vaccine Ltd, a sister concern of Incepta Pharmaceuticals Ltd has introduced tetanus vaccine in Bangladesh market with the brand name of Vaxitet. Tetanus vaccine (Vaxitet) will help to save millions of neonates and mother from life threatening tetanus. Vaxitet will be available to patients at affordable price in 0.5 ml ampoule and can be given intramuscularly.
Incepta Vaccine Ltd, a sister concern of Incepta Pharmaceuticals Ltd has introduced life saving rabies vaccine in Bangladesh market with brand name Rabix-vc. In Bangladesh, more than 2000 people die from rabies every year which is highest in the world. But acute shortage, counterfeit, inadequate availability of imported vaccine, doubt with potency of vaccine due to improper maintenance of cold chain during transportation and high cost are major drawback to prevent this deadly disease. Now Incepta has stepped forward to manufacture high quality and affordable rabies vaccine in Bangladesh market. Rabix-vc is marketed in 1 ml vial which can be given intramuscularly.
Every year Bangladesh Government has to purchase more than 90 million doses of different vaccines to initiate the Expanded Program on Immunization (EPI) for children of 0-1 year. In private sector other than EPI, every year more than 30 million doses are required. This quantity is increasing day by day. Huge foreign currency is spent to purchase these vaccines. Since its inception, Incepta is always trying to fill up the therapeutic gap by providing the newer and modern needful medicines. Incepta Vaccine Ltd, a sister concern of Incepta Pharmaceuticals Ltd introduced first vaccine Vaxphoid, a vaccine to prevent typhoid fever which is one of the major causes of childhood death in Bangladesh.
Incepta Vaccine Ltd is the first Human vaccine company in Bangladesh, developed with primary objective to provide preventive medicine to vast majority of population at an affordable price. It is a new state of the art facility to manufacture vaccine in Bangladesh for the first time. The facility is fully compliant with WHO GMP guidelines. It has an advanced fully GMP compliant R&D facility which can independently handle several projects simultaneously involving bacteria and virus. Incepta has well equipped microbiology and chemical laboratory, a large lab animal house covering around 24,000 square feet area which has bio safety level 2 standard for testing of various vaccines.
Incepta has unique cold chain system to maintain the required temperature in every step of filling to packaging. It has well designed spacious several cold rooms to store raw materials and finished products. It has 4 cold rooms of temperature within 2-8 °C and 2 cold rooms of temperature -20 °C to store raw materials and finished products.
In near future, Rabies, Tetanus, Hepatitis B, Polio, Measles, Rubella, Tetanus antitoxin, Rabies immunoglobulin, Pentavalent and all other necessary vaccines will be manufactured. Incepta Vaccine Ltd is committed to provide all high tech life saving vaccines to the people of Bangladesh at affordable price.
Incepta Vaccine Limited, a sister concern of Incepta Pharmaceuticals Ltd received GMP certificate from the Directorate General of Drug Administration, Ministry of Health & Family welfare, Government of the Peoples Republic of Bangladesh.
Incepta received new license, Incepta Vaccine Ltd, to manufacture vaccine for the first time in Bangladesh.
03 June 2024
Murdoch Children's Research Institute (MCRI) and Incepta Pharmaceuticals, based in Bangladesh, have embarked on a collaboration aimed at bringing life-saving innovation to the global healthcare landscape.

MCRI has announced the signing of a non-exclusive licensing agreement with Incepta Pharmaceuticals, marking another milestone in its journey to reduce the impact of rotavirus, the most common cause of severe diarrhoea in infants and young children worldwide.
RV3-BB, adapted and developed from a naturally occurring human strain of rotavirus discovered in Melbourne by Professor Ruth Bishop AC and colleagues inthe early 1980’s , offers early protection against dehydrating diarrhoea from birth. The RV3-BB vaccine, intended for neonatal or infant dosing schedules as part of routine Expanded Program on Immunization (EPI) vaccinations, has the potential to save hundreds of thousands of children’s lives annually.
In Bangladesh alone, where more than two million babies are born every year, the impact of RV3-BB could be profound. Globally, rotavirus claims the lives of about 450,000 children under the age of five annually, making the need for accessible and effective vaccines urgent.
MCRI Professor Andrew Steer, Director of Infection, Immunity and Global Health said, “This collaboration represents a significant step forward in our mission to protect vulnerable children from the effects of rotavirus infection. By joining forces with Incepta Pharmaceuticals, we are poised to make RV3-BB readily available to communities in need worldwide.
“MCRI's relentless pursuit of accessible and scalable vaccine solutions underscores our commitment to global health equity.”
Abdul Muktadir, Chairman & Managing Director of Incepta Pharmaceuticals said, "We are proud to partner with MCRI in this groundbreaking endeavour. Together, we are committed to leveraging our expertise and resources to ensure that rotavirus vaccine (RV3-BB) reaches the millions of childrenwho need it most, safeguarding the health of future generations."

Abdul Muktadir, Chairman & Managing Director, Incepta Pharmaceuticals Ltd.
With Incepta Pharmaceuticals' established reputation as a leading pharmaceutical company in Bangladesh, equipped with the capacity to produce vaccines at scale, RV3-BB vaccine is poised to make a transformative impact on childhood health worldwide.
Media Contact:
Incepta Pharmaceuticals ltd.
Email: info@inceptapharma.com
About Murdoch Children’s Research Institute:
Murdoch Children's Research Institute is the largest child health research institute in Australia committed to making discoveries and developing treatments to improve child and adolescent health in Australia and around the world. They are pioneering new treatments, trialling better vaccines and improving ways of diagnosing and helping sick babies, children and adolescents. It is one of the only research institutes in Australia to offer genetic testing to find answers for families of children with previously undiagnosed conditions.
About Incepta Pharmaceuticals:
Incepta Pharmaceuticals Ltd is a leading pharmaceutical company based in Bangladesh, specializing in the development, manufacturing, and marketing of a wide range of pharmaceutical products since 1999. With a strong commitment to healthcare improvement, Incepta has been at the forefront of producing innovative vaccines and medications that meet the highest standards of quality and efficacy.
18 Jan 2023
Imperial College London, the world's leading medical education and research institute, has expressed interest in working with Incepta Pharmaceuticals, a renowned vaccine manufacturing of the country. Professor Robin Shattock, head of the research team at the Department of Infectious Diseases, Imperial College, London, visited Vaccine and Injectable plants of Incepta at Savar and Dhamrai . He expressed his great appreciation and said that Incepta’s management is very commendable. Mr. Abdul Muktadir, Chairman and Managing Director of Incepta Pharmaceuticals Ltd., was present during the on-site inspection of vaccine pants. At the end of the visit, mRNA vaccine expert Professor Robin John Shattock reiterated to support Incepta in technology and research to further develop its ability towards a variety of life-saving new vaccines.
Mr Abdul Muktadir, chairman and managing director of Incepta Pharmaceuticals Ltd. stated that Professor Robin John Shattock of Imperial College has helped Incepta to develop protein subunit vaccine in the past. In this continuation, he will also provide technical assistance in the development of other vaccines.
He also added that Incepta is now involved in the development of therapeutic vaccines. We will now jointly research in both replicating and non-replicating mRNA vaccines. We will also participate in future research on mRNA and therapeutic vaccines. He said, Professor Robin is a world-famous scientist. There are many scientists of this country who will be trained with his help and new areas of research will be opened. We have already trained two scientists of Incepta from Imperial College. Many more will be trained soon. This will also open the door to manufacture basic materials (seeds) of vaccine in Bangladesh. We believe that a new era has dawned in the field of research on vaccines and drugs. By doing this it will be possible to bring better benefits to the people.
Jan 09, 2023
Sarah Gilbert, a vaccinologist and co-inventor of AstraZeneca coronavirus vaccine, visited the office of Incepta Pharmaceuticals today.
Gilbert is a professor of vaccine science at the Jenner Institute, Oxford and Nuffield Department of Clinical Medicine of the University of Oxford. She led the development of the Oxford-AstraZeneca vaccine for Covid-19.
She was awarded the prestigious Albert Medal by the Queen in 2021 and Damehood by the Princess Royal at Windsor Castle in 2022 for her contribution to developing the Oxford-AstraZeneca vaccine.
Abdul Muktadir, chairman and managing director of Incepta Pharmaceuticals, and Hasneen Muktadir, vice-chairman, welcomed Gilbert at the Incepta head office in Tejgaon, Dhaka.
Future challenges of vaccine production in Bangladesh, availability of vaccines to common people, and capacity of vaccine production were discussed during her visit. Various aspects of modern and advanced technologies in vaccine production were deliberated and Gilbert gave her guidance on how Incepta can incorporate these modern technologies, said the drug maker.
2 August 2022
A seminar titled 'An Effort to Build a Cervical Cancer Free Bangladesh' and the unveiling of Incepta's new vaccine Papillovax were held at the capital's Pan Pacific Sonargaon Hotel on Tuesday, August 2.
The seminar discussed the need for HPV vaccine (human papillomavirus) in the country and how regular screening and introduction of HPV vaccine (human papillomavirus) Papillovax can contribute to achieving a cervical cancer free country.
Dr. expert doctor in the seminar. TA Chowdhury and National Professor Shahla Khatun delivered speeches as chief guests. The seminar was presided over by the president of OGSB (Obstetrical and Gynecological Society of Bangladesh) Professor Dr. Ferdowsi Begum. Professor Dr. The seminar started with welcome address by Gulshan Ara.
Former Chairman of Gynecology Oncology Department of Bangabandhu Sheikh Mujib Medical University Prof. Dr. Sabera Khatun and Manager (MSD) of Incepta Pharmaceuticals Farhana Laiju presented on the subject in the seminar. Scientific Secretary of OGSB, Professor Sheikh Jinnat Ara Nasreen moderated the excellent question and answer session.
Incepta Pharmaceuticals Ltd. expressed thanks on behalf of Incepta at the seminar. Executive Director Dr. EH Arefin Ahmed. Finally, Incepta officially unveiled PapilloVax, heralding a new era in cervical cancer prevention.
To eliminate cervical cancer, the HPV vaccine is the first step. There was a huge gap in this vaccine in Bangladesh for the past few years. Incepta Vaccine Ltd., the country's first vaccine manufacturing company, has come forward to fill this gap. and started marketing the country's first cervical cancer vaccine, Papillovax. It is a matter of pride for the country, which will pave the way to eradicate cervical cancer from the country.
18 July 2022
Incepta Vaccine Limited launched its cervical cancer vaccine "Papilovax" for the first time in Bangladesh as a local company.
Papilovax protects women against cervical cancer by countering the HPV virus responsible for the disease.
Women 9-45 years in age should receive three doses of Papilovax. The second dose should be taken one month after the first dose and the third dose should be received six months after that.
Completing the full course of this vaccine will reduce the risk of developing cervical cancer throughout a woman's lifetime.
Cervical cancer is the second deadliest among different types of cancers that cause female deaths in Bangladesh. Every year more than 10,000 women die of cervical cancer in the country and more than five crore women are at risk.
Papilovax is marketed in modern pre-filled syringes. The full dose in pre-filled syringes is manufactured in an aseptic environment and marketed at controlled temperatures in fully sterile packaging.
Today 16th August, Monday at 3.00 pm an agreement has been signed between the Sinopharm of China, Ministry of Health and Family Welfare, People’s Republic of Bangladesh and Incepta Vaccine Ltd, renowned vaccine manufacturing company of the country, at the auditorium of Bangladesh College of Physicians and Surgeons (BCPS), Mohakhali Dhaka in order to produce the Sinopharm covid vaccine in Bangladesh.
Honorable Minister of Health and Family Welfare Mr. Zahid Maleque MP was the Chief Guest at the signing ceremony. Honorable Foreign Minister Dr. A. K. Abdul Momen MP was present as the guest of honor.
Mr. Masud Bin Momen, Senior Secretary, Ministry of Foreign Affairs, His Excellency, People’s Republic of China Li Jiming, Major General Md, Mahbubur Rahman, Director General, Directorate General of Drug Administration (DGDA), and Mr Abdul Muktadir, Chairman of Incepta Vaccine Ltd and Incepta Pharmaceuticals Ltd were also present in the program as Special guests. Prof. Dr. Abul Bashar Mohammad Khurshid Alam, Director General, Directorate General of Health Services (DGHS) gave a welcome speech. Mr. Lokman Hossain Miah, Senior Secretary, Health Service Division, Ministry of Health and Family Welfare presided over the function.
In addition, Counselor of the Department of Asia Affairs of the Ministry of Foreign Affairs of China Ji Rong and the Chairman of Sinopharm Mr Liu Jingzhen were connected online from Beijing and delivered speech as special guests. Mr. Fu Qiang, Chief Engineer of Sinopharm gave the welcome speech on behalf of Sinopharm.
Aiming to co-produce the China's Sinopharm vaccine in Bangladesh, the Memorandum of Understanding was signed by Honorable Minister Mr. Zahid Malek MP, His Excellency, People’s Republic of China Mr. Li Jiming and Abdul Muktadir, Chairman of Incepta Vaccine Ltd and Incepta Pharmaceuticals Ltd on behalf of Ministry of Health and Family Welfare, Sinopharm and Incepta Vaccine Ltd respectively.
Speakers at the event said that a new direction has been unveiled with this agreement in the production of covid vaccine in Bangladesh in this Mourning August. It was expected that through this agreement, Incepta Vaccine Ltd, would produce the Sinopharm Vaccine very soon.
For world cup football 2018, Incepta Vaccine Ltd sponsored a prediction game in the daily newspaper Prothom Alo which continued throughout the month. During this event, two winners had been selected in every week and finally total eight winners have been selected.
In the last 5th August, 2018 an award ceremony had been arranged with the all winners in the premises of the head office of Incepta Pharmaceuticals Ltd. All winners had been appreciated with attractive gifts. During this time, all participants gave their gratitude to the Prothom Alo and Incepta. The ceremony had packed up with the photo session. The COO of Prothom Alo and the General Manager of Incepta Pharmaceuticals were present in the program.
The day was observed with colorful rally and different awareness programs
Like every year, Incepta Vaccine Ltd. observed World Hepatitis Day 2018 on July 28th in Bangladesh with the collaboration of National Liver foundation of Bangladesh.
To observe the day, National Liver Foundation of Bangladesh (NLFB) conducted different awareness-building events where the campaign was “Find the Missing Millions”. The theme of the day was “Eliminate Hepatitis” by 2030’.
The colorful rally includes Roller skates, Horse carts and Motorbike started from Manik Mia Ave, crosses the main road of Dhaka city and ended at Daily star Auditorium, Kawranbazar. Doctors, Patients, members of Youth Club of Bangladesh and many University and college students actively participated in the rally and other events of the day. Moreover, A seminar was held at the Daily star Auditorium. National Professor Jamilur Raja Choudhury, Chairman of National Liver Foundation of Bangladesh, chaired the seminar. Ekushey Padak winner Prof. MirzaMazharul Islam, renowned surgeon & member Board of Adviser of the foundation was the chief guest. Prof. Mohammad Ali, Secretary General, NLFB delivered the keynote speech. He highlighted the various ongoing activities of the foundation and awareness events especially NOhep network and Find the Missing Million campaign in Bangladesh.
Incepta Vaccine Ltd. observed World Rabies Day 2017 on September 28 in Bangladesh. The day was observed with full of events e.g. rally, discussion on rabies and its preventive measures in different institutes with presence of doctors, nurses and other healthcare professionals.
"Rabies: Zero by 2030" with this theme the rally was started from Infectious Disease Hospital (IDH), Dhaka and ended at Krishibid Institute at Farmgate, Dhaka with the participation of Dr. Syad Ahsan Touhid, Sr. Consultant (Acting), IDH.
Besides, the day was also observed at Mohammad Ali Hospital, Bogra; Abdul Malek Ukil Medical College, Noakhali etc. sponsored by Incepta Vaccine Ltd. The MPOs, Area Managers and Regional Managers of Incepta dressed with the T-Shirt designed with logo of rabies day and Incepta.
ELIMINATE HEPATITIS, NOhep Drive 2017
Incepta vaccine ltd observed this day with the collaboration of the National Liver Foundation of Bangladesh by conducting different events throughout the country to spread the knowledge of Hepatitis B and eliminate it. This year the day was observed with the theme of ELIMINATE HEPATITIS,NOhep Drive.
A nationwide viral hepatitis awareness campaign throughout Bangladesh named as ELIMINATE HEPATITIS Nohep Drive 2017 was launched by the National Liver Foundation of Bangladesh (NLFB) on July 10, 2017, on the eve of World Hepatitis Day 2017 with the active participation of Incepta. This is in the light of the global viral hepatitis movement “NOhep” of World Hepatitis Alliance.
This event was started at The Central ShaheedMinar (Martyr’s Monument) at capital city Dhaka. NOhep branded Van started to move from Dhaka carrying volunteers, leaflets, banners, audiovisual presentations, viral hepatitis awareness questionnaires, posters, and other NOhep merchandise.
This Nohep van traveled all the EIGHT DIVISIONAL HEADQUARTERS of Bangladesh, starting from Dhaka>Rongpur>Rajshahi>Mymensingh>Sylhet>Chittagong>Barisal and finally to Khulna.
These 12 days long tour traveled more than 2000 kilometers of city and rural areas of the country. Conveyed messages to people of different walks of life, cultures and social status, with the aim of reaching the grass-root level. Distributed more than 30,000 leaflets, It also transmits messages to educational institutes, religious places, village markets, and public gatherings.
The program designed to touch most of the parts of Bangladesh to disseminate the message of NOhep campaign. It will act as groundwork for the elimination of viral hepatitis in Bangladesh.
Incepta Vaccine Ltd has launched its vaccine bulk manufacturing facility in Savar, outside the capital. Honorable health Minister Mohammed Nasim, MP inaugurated the facility on Dewan Idris Road in Zirabo on Thrusday, 28 July.
An internationally bulk manufacturing facility has been developed providing the best environmental conditions for performing the operations. The facility can manufacture both Bacterial and Viral bulk antigen. These make us less dependent on import and help to become a self-sufficient vaccine manufacturing country.
Addressing the inaugural ceremony, the minister said now Bangladesh is able to export medicines to 90 countries because of their high quality. Bangladesh is now capable of manufacturing life saving vaccine and successfully reduces the dependency on imported vaccines. ‘We will take the initiatives to get the WHO authorization which is needed for export of vaccines.’ Nasim said.
Director General of Drug Administration, Major General Md Mustafizur Rahman, Secretary General of Bangladesh Association of Pharmaceutical Industries, SM Shafiuzzaman were also present there as special guest.
Public-private collaboration among Incepta Vaccine Ltd, IVI, and icddr. Following technology transfer from IVI to Incepta Vaccine Ltd, clinical trials to start in Dhaka, Bangladesh
The International Vaccine Institute (IVI), International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), and Incepta Vaccine Ltd. announced today initiation of a clinical trial for Cholvax®, an inactivated oral cholera vaccine, in Dhaka, Bangladesh.The three groups are collaborating on its clinical development in order to register the vaccine in Bangladesh, the first oral cholera vaccine to be registered in the country.
The clinical trial will assess vaccine safety and immunogenicity in 2,052 people 1-45 years of age in Dhaka. As the trial sponsor, IVI will lead this research effort in collaboration with icddr,b, an international health research organization based in Bangladesh, and will also monitor the trial for quality assurance and provide technical support in data management and evaluation of the participants safety as well as immune response to the vaccine. Incepta, the first human vaccine manufacturer in Bangladesh, will be responsible for manufacturing and quality control of clinical lots of Cholvax® for the trial. The trial is supported by the Bill & Melinda Gates Foundation.
The partnership between IVI and Incepta began in July 2014 when IVI transferred the technology for oral cholera vaccine production and quality control methods to the company. Both IVI and Incepta are committed to developing the vaccine for the public sector in Bangladesh where a high burden of cholera exists. This is the first collaboration between IVI and Incepta. The two organizations are also working together on the development of a typhoid conjugate vaccine.
The vaccine is a bivalent killed whole-cell oral cholera vaccine given orally in two doses over a 14-day interval and intended for use in people one year old and above. It was originally reformulated by IVI and developed through an international public-private partnership led by IVI, with partners from Sweden, India, Vietnam, and South Korea, and with support from the governments of South Korea and Sweden, and the Bill & Melinda Gates Foundation.
“The collaboration with Incepta and icddr,b will result in a vaccine that can reduce needless suffering of so many people from a debilitating and potentially fatal disease,” said Dr. Jerome Kim, IVI’s Director General, “This collaboration is critical to achieving our goal of making vaccines available and accessible for the world’s most vulnerable and impoverished people.”
Incepta Vaccine Ltd. observed World Hepatitis Day 2015 on July 28th in Bangladesh. The day was observed with full of events in relation to create awareness to prevent viral hepatitis.
“Prevent Hepatitis, It’s up to you” with this slogan, a Cycle Rally has been initiated from the Central Shahid Minar and circled major roads of Dhaka city. Mr.Mahbubey Alam, Attorney General, Government of the People’s Republic of Bangladesh, Mr. Sayeed Khokon, Honorable Mayor, Dhaka City (South) and Prof. Mohammad Ali, Secretary General, National Liver Foundation of Bangladesh inaugurated the rally and wished to provide all out support for awareness activities for viral hepatitis.
Special program was broadcasted in television & different topics were transmitted in popular FM Radio whole day. The importance of prevention of viral hepatitis was also published in social media and in the newspaper. Posters, banners, leaflets were widely circulated on this auspicious day.
Vaccination for influenza and meningitis are two mandatory prerequisite for all Bangladeshi hajj pilgrims. Government of Bangladesh ensured vaccination of these two vaccines one month prior to the departure for Saudi Arabia.
In this year 2015, Incepta Vaccine Ltd is very much delighted to provide supply of influenza and meningitis vaccine to Government of Bangladesh for the hajj pilgrimages. Incepta Vaccine Ltd manufactured Influenza vaccine by the name of “Influvax” and Meningococcal polysaccharide vaccine by the name of “Ingovax” for meningitis. It has been a pride for the Incepta to be a part of this novel activity. Incepta pray for the safety and wellbeing of all Bangladeshi hajj pilgrim
Antivenom injection is the preparation of snake venom antiserum. In Bangladesh, about 8000 snake bites occur annually with more than 20% mortality. Snake bite causes serious systemic or neurological complications such as bleeding disorder leading to fatal hemorrhage, permanent disability or death. Treatment of snake bite is neglected and is left to traditional ozha or kaviraj which lead to death. Snake venom antiserum is the mainstay of treatment after snake bite. But in Bangladesh, this life saving antiserum was not available everywhere in every season due to there is no local manufacturer, lack of cold chain system. Now Incepta Vaccine Ltd has introduced snake venom antiserum for the first time in Bangladesh with the brand name of Antivenom. Antivenom is manufactured by applying high tech lyophilization technique so that it can be stored at room temperature and highly stable. Antivenom is available in 10 ml in vial after reconstitution that should be given as infusion.
Influenza vaccine protects against influenza viruses during influenza season. Flu virus causes respiratory infection which passes through the air and enters the body through nose or mouth. It spreads around the world in a yearly outbreak resulting in about 3 to 5 million cases. Flu leads to severe complication for chronically ill patient like Asthma, COPD, Diabetes, Heart disease. Flu can easily be prevented by vaccination before starting of every flu season. In Bangladesh, this life saving vaccine was imported, which was not available everywhere in every season. Now Incepta Vaccine Ltd has introduced Influenza vaccine for the first time in Bangladesh with the brand name of Influvax. Influvax vaccine is manufactured with the WHO recommended flu viruses (A/California/7/2009 (H1N1), A/Texas/50/2012 (H3N2), B/Massachusetts/2/2012 ) for northern hemisphere that are recommended for our country. Influvax is available in 0.5 ml vial that can be given intramuscularly.
Anti rabies vaccine is recommended as soon as possible after exposure to rabid animal to prevent rabies. However, in severe cases like WHO category-III bite only anti rabies vaccine is not enough to save life. In this case rabies immunoglobulin is recommended by WHO to ensure immediate protection. In Bangladesh, this life saving rabies immunoglobulin was imported, which was expensive and not available everywhere. Now Incepta Vaccine Ltd, a sister concern of Incepta Pharmaceuticals Ltd has introduced Rabies Immunoglobulin first time in Bangladesh with the brand name of Rabix-IG. Rabix-IG is available in 5 ml vial that can be given intramuscularly.
Tetanus antitoxin provides immediate protection against tetanus in tetanus prone wounds that can be happened in our life any time. About 73% cases of tetanus infection are caused by acute injuries or wounds. Bangladesh was fully dependent on imported tetanus antitoxin to prevent this deadly disease. Now Incepta Vaccine Ltd, a sister concern of Incepta Pharmaceuticals Ltd has introduced Tetanus antitoxin first time in Bangladesh with the brand name of Vaxitet-IG. Vaxitet-IG is available in 1 ml vial that can be given intramuscularly or subcutaneously.
Hepatitis B virus infection is one of the major burdens for Bangladeshi people. More than 10 million people are suffering from hepatitis B virus infection. Moreover, Bangladesh was fully dependent on imported vaccine to prevent this deadly infectious disease. Now Incepta Vaccine Ltd, a sister concern of Incepta Pharmaceuticals Ltd has introduced Hepatitis B vaccine first time in Bangladesh with the brand name of Hepa-B. Hepa-B is available in vial of 0.5 ml for pediatric and 1 ml for adult that can be given intramuscularly.
The first ever Human Vaccine manufacturing plant of Incepta Vaccine Ltd was officially inaugurated by the Honorable Health & Family Welfare Minister Prof. Dr. A. F. M. Ruhal Haque MP. Honorable Senior Secretary of Health & Family Welfare Ministry Md. Humayun Kabir, National Professor M. R. Khan, Director General of Drug Administration Major General Md. Abul Kalam Azad, Additional Director General of Directorate General of Health Services Prof. Dr. A.F.M. Saiful Islam and Director of Primary Health Care and Line Director of MNC & AH Dr. Syed Abu Jafar Md. Musa were present in the inauguration program as special guests. Abdul Muktadir, Managing Director of Incepta Vaccine Ltd, Mrs. Hasneen Muktadir, Director, Planning & Commercial, Director Jahiruddin Mahmud, Abdul Mukit Majumdar, Abdur Rashid Majumdar, Mahbubul Karim, Director, Technical Operations and other senior officials of Incepta were also present in the program.
In his speech, honorable Minister of Health & Family Welfare Prof. Dr. A. F. M. Ruhal Haque said “I am appreciating such initiative of Incepta Vaccine Ltd. At present our Government needs to spend several hundred crore Taka to import the necessary vaccines for EPI program. But, now we can save huge amount of foreign currency for such initiative of Incepta Vaccine Ltd.” Honorable Minister also visited the newly constructed 3 stored vaccine facilities which consist of 75000 square-feet, animal house which is required to ensure the efficacy of manufactured vaccines and also cold chain system which is important to maintain the efficacy of vaccine. He appreciated much to see such a world class vaccine plant and its facilities.
National Professor M. R. Khan said “Incepta has been supplying medicine of supreme quality from its inception. Their initiative makes a remarkable appreciation to reduce the dependency on foreign medicine.”
Abdul Muktadir, Managing Director of Incepta Vaccine Ltd said “in the near past we had to import essential vaccines from foreign countries. But now we are manufacturing vaccines in our own country. We have constructed a totally new and enormous vaccine plant according to the guidelines of WHO GMP.” He also said “the annual production capacity of our vaccine plant is about 18 crore vials which will not only fulfill the annual demand of EPI program but also provide us the opportunity to export these vaccines in the foreign countries.”
In conclusion, Mr. Muktadir promised to make all these high-tech and life saving vaccines available and affordable for the people of our country and sought overall assistance from the Government of Bangladesh for such endeavor.
Incepta Vaccine Ltd is the first Human vaccine company in Bangladesh, developed with primary objective to provide preventive medicine to vast majority of population at an affordable price. It is a new state of the art facility to manufacture vaccine in Bangladesh for the first time. The facility is fully compliant with WHO GMP guidelines. It has the following unique features:
Incepta Vaccine Ltd can produce any dose size from 0.1ml to 15 ml, with 4 different products simultaneously and has capacity to fill more than 2,40,000 ampoules and 3,60,000 vials per day. The yearly capacity is 180 million vials and ampoules. To conduct the immunization program of EPI, yearly demand of vaccine is 21 million vials and other than EPI yearly demand is 10 million vials which is increasing day by day. The production capacity of this facility is enough to meet the local demand and export.
Incepta Vaccine Ltd is now manufacturing and marketing Typhoid vaccine (Vaxphoid), Rabies vaccine (Rabix-vc) and Tetanus vaccine (Vexitet). In near future Hepatitis B, Polio, Measles, Rubella, Tetanus antitoxin, Rabies immunoglobulin, Pentavalent and all other necessary vaccines will be manufactured. Incepta Vaccine Ltd is committed to provide all high tech life saving vaccines to the people at affordable price.
Incepta Vaccine Ltd, a sister concern of Incepta Pharmaceuticals Ltd has introduced tetanus vaccine in Bangladesh market with the brand name of Vaxitet. Tetanus vaccine (Vaxitet) will help to save millions of neonates and mother from life threatening tetanus. Vaxitet will be available to patients at affordable price in 0.5 ml ampoule and can be given intramuscularly.
Incepta Vaccine Ltd, a sister concern of Incepta Pharmaceuticals Ltd has introduced life saving rabies vaccine in Bangladesh market with brand name Rabix-vc. In Bangladesh, more than 2000 people die from rabies every year which is highest in the world. But acute shortage, counterfeit, inadequate availability of imported vaccine, doubt with potency of vaccine due to improper maintenance of cold chain during transportation and high cost are major drawback to prevent this deadly disease. Now Incepta has stepped forward to manufacture high quality and affordable rabies vaccine in Bangladesh market. Rabix-vc is marketed in 1 ml vial which can be given intramuscularly.
Every year Bangladesh Government has to purchase more than 90 million doses of different vaccines to initiate the Expanded Program on Immunization (EPI) for children of 0-1 year. In private sector other than EPI, every year more than 30 million doses are required. This quantity is increasing day by day. Huge foreign currency is spent to purchase these vaccines. Since its inception, Incepta is always trying to fill up the therapeutic gap by providing the newer and modern needful medicines. Incepta Vaccine Ltd, a sister concern of Incepta Pharmaceuticals Ltd introduced first vaccine Vaxphoid, a vaccine to prevent typhoid fever which is one of the major causes of childhood death in Bangladesh.
Incepta Vaccine Ltd is the first Human vaccine company in Bangladesh, developed with primary objective to provide preventive medicine to vast majority of population at an affordable price. It is a new state of the art facility to manufacture vaccine in Bangladesh for the first time. The facility is fully compliant with WHO GMP guidelines. It has an advanced fully GMP compliant R&D facility which can independently handle several projects simultaneously involving bacteria and virus. Incepta has well equipped microbiology and chemical laboratory, a large lab animal house covering around 24,000 square feet area which has bio safety level 2 standard for testing of various vaccines.
Incepta has unique cold chain system to maintain the required temperature in every step of filling to packaging. It has well designed spacious several cold rooms to store raw materials and finished products. It has 4 cold rooms of temperature within 2-8 °C and 2 cold rooms of temperature -20 °C to store raw materials and finished products.
In near future, Rabies, Tetanus, Hepatitis B, Polio, Measles, Rubella, Tetanus antitoxin, Rabies immunoglobulin, Pentavalent and all other necessary vaccines will be manufactured. Incepta Vaccine Ltd is committed to provide all high tech life saving vaccines to the people of Bangladesh at affordable price.
Incepta Vaccine Limited, a sister concern of Incepta Pharmaceuticals Ltd received GMP certificate from the Directorate General of Drug Administration, Ministry of Health & Family welfare, Government of the Peoples Republic of Bangladesh.
Incepta received new license, Incepta Vaccine Ltd, to manufacture vaccine for the first time in Bangladesh.
Vaccine Division 40 Shahid Tajuddin Ahmed Sarani Tejgaon I/A, Dhaka-1208. Bangladesh
info@inceptavaccine.com
+88-0222-4498130-7,
+88-02-8891688 - 703
© 2025 . Incepta Pharmaceuticals Ltd. All rights reserved.
Site By DIGITA iNTERACTIVE
