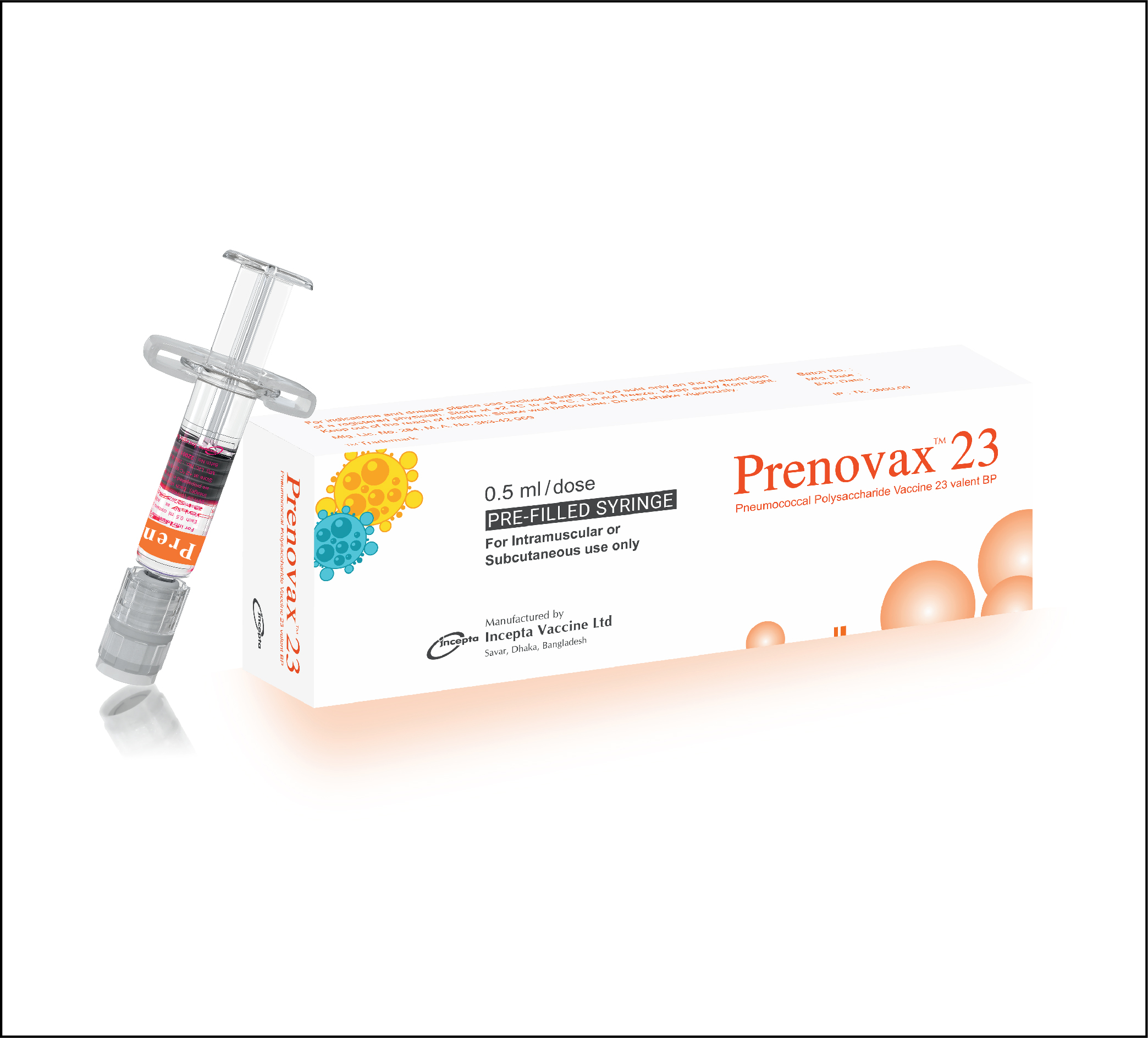
Presentation:
Prenovax 23: Each 0.5 ml contains Pneumococcal Polysaccharide Vaccine 23 valent BP consisting of purified capsular polysaccharides of Streptococcus pneumoniae serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F of 25 μg each.
Description:
Prenovax 23 is a sterile liquid vaccine consisting of a mixture of purified capsular polysaccharides from Streptococcus pneumoniae serotypes (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F). No preservatives are added. This vaccine is supplied as a single-dose, pre-filled syringe. This vaccine is presented as a clear and colorless solution.
Indications:
Prenovax 23 is a vaccine indicated for active immunization for the prevention of pneumococcal disease caused by the 23 serotypes contained in the vaccine (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F). Prenovax 23 is approved for use in persons 50 years of age or older and persons aged ≥ 2 years who are at increased risk for pneumococcal disease.
Dosage and Administration:
Dose: Single dose (0.5 mL).
Administration:
This vaccine is for intramuscular or subcutaneous injection (intramuscularly recommended) and the preferred administration site is the deltoid muscle of the lateral upper arm or lateral mid thigh. Intravascular and intradermal injection is prohibited.
Revaccination:
Persons at high risk (splenectomy) who were previously vaccinated over 5 years or with significant decreasing antibody titers (e.g., nephrotic syndrome, renal failure and organ transplantation) are recommended to revaccinate against pneumococcal diseases.
Children under 10 years old with nephrotic syndrome, splenectomy or sickle cell diseases are recommended to revaccinate 3-5 years later after the initial vaccination.
The immune persistence of this product has so far not demonstrated by any clinical study. The recommendations for revaccination above are compiled based on the recommendations for subsequent doses prescribed in the Prevention of Pneumococcal Diseases-Recommendations by the Advisory Committee on Immunization Practices.
Side Effects:
The most common adverse reactions: injection-site pain/soreness/tenderness, injection-site swelling/induration, headache, injection-site erythema, asthenia/fatigue and myalgia.
The incidence rates of adverse reactions reported in clinical trials, according to the guidance on classifications of adverse events are classified as: very common (≥10%), common (≥1% to <10%), uncommon (≥0.1% to <1%), rare (≥0.01% to <0.1%), and very rare (<0.01%) as follows:
Very common:
Local reaction(s): pain.
Common:
Local reactions: redness, swelling, itching. Systemic reactions: fever, fatigue, headache,
Uncommon:
Local reaction(s): induration.
Systemic reactions: vomiting, rash, allergy.
Precautions:
Do not administer intracutaneously or intravenously, and ensure the syringe needle is not puncturing blood vessels during inoculation.
The vaccine shall be administered with caution to nursing women or individuals with family or individual history of convulsion, history of epilepsy and allergic diathesis.
Check if the package, container, label, appearance and expiration date of the vaccine follow corresponding requirements before administration. Do not use the vaccine in case that any crack is observed in the container, loosened stopper, detached label, particulate matter or discoloring inside the container, etc. Do not use the vaccine after the expiration date.
Use immediately after unsealing. A single human dose shall be used up each time according to prescribed information.
Appropriate monitoring, medical care and rescue measures should be readily available in case of rare hypersensitivity reactions during vaccination. The recipient shall be observed for at least 30 minutes on site following injection.
If allergic reactions occur after vaccination, please visit the vaccination site or hospital in time. Do not freeze. Discard the vaccine if it has been frozen.
Defer vaccination in persons with moderate or severe acute illness.
Caution and appropriate care should be exercised in administering vaccine to individuals with severely compromised cardiovascular and/or pulmonary function in whom a systemic reaction would pose a significant risk.
This vaccine does not replace the need for penicillin (or other antibiotic) prophylaxis against pneumococcal infection. In patients who require penicillin (or other antibiotic) prophylaxis against pneumococcal infection, such prophylaxis should not be discontinued after vaccination.
Persons who are immunocompromised, including persons receiving immunosuppressive therapy, may have a diminished immune response.
The vaccine may not be effective in preventing pneumococcal meningitis in patients who have chronic cerebrospinal fluid (CSF) leakage resulting from congenital lesions, skull fractures, or neurosurgical procedures.
Contraindications:
This vaccine should not be administered to:
Individuals with allergic reactions to any component of the vaccine.
Individuals with encephalopathy, uncontrolled epilepsy, or other progressive diseases of the nervous system.
Individuals with fever, acute infection, or chronic diseases at the acute stage.
Only if clearly needed, otherwise revaccination within 3 years is not recommended.
Use in Pregnancy and Lactation:
Pregnancy
Administering this vaccine in pregnant women is not recommended. Administration of the vaccine in this population is determined by doctors based on the risk faced with the potential recipients.
Lactation
Administration of the vaccine in nursing mothers should be determined by doctors with cautions.
Pediatric Use
The vaccine is not approved for use in children less than 2 years of age. Children in this age group do not develop an effective immune response to the capsular types contained in this polysaccharide vaccine.
The ACIP has recommendations for use of this vaccine in children 2 years of age or older, who have previously received pneumococcal vaccines, and who are at increased risk for pneumococcal disease.
Over Dosage:
Not applicable
Storage:
Keep out of the reach and sight of children.
Store at +2oc to 8oc. Transportation should also be at +2oc to +8oc. Do not freeze. Discard vaccine if frozen.
Protect from light.
Commercial Pack:
Prenovax 23: Each box contains 0.5 ml sterile liquid of Pneumococcal Polysaccharide Vaccine 23 valent BP in a prefilled syringe and 2 needles.



.png)