Presentation:
KhuraVax Vet: Each dose of vaccine contains Trivalent Foot and Mouth Disease Inactivated antigens O, A and Asia-1 strains, adjuvanted with mineral oil. Potency of the vaccine is ≥ 3 PD50/dose.
Description:
Food and Mouth Disease (FMD) vaccine is an inactivated, trivalent viral vaccine containing virus type O, A & Asia-1. The inactivated antigen is purified, concentrated, adjuvanted and ready for use. It is adjuvanted with mineral oil and Thiomersal. Mineral oil is added as adjuvant instead of Aluminium Hydroxide to ensure enhanced immune response and stability. World Animal Health Organization (OIE) recommended tissue culture FMD vaccine that has high immunogenicity and safety profile. It acts by stimulation of predominantly humoral immune response in the vaccinated animals. Potency of the vaccine is ≥3 PD50/dose. Protective immune response is induced 14 days after vaccination.
Indications:
• Vaccine is recommended for cattle, goat, sheep and buffaloes as a prophylactic measure against Foot
and Mouth Disease
• For disease control by routine vaccination in endemic area
• For limiting the spread of the disease during outbreak
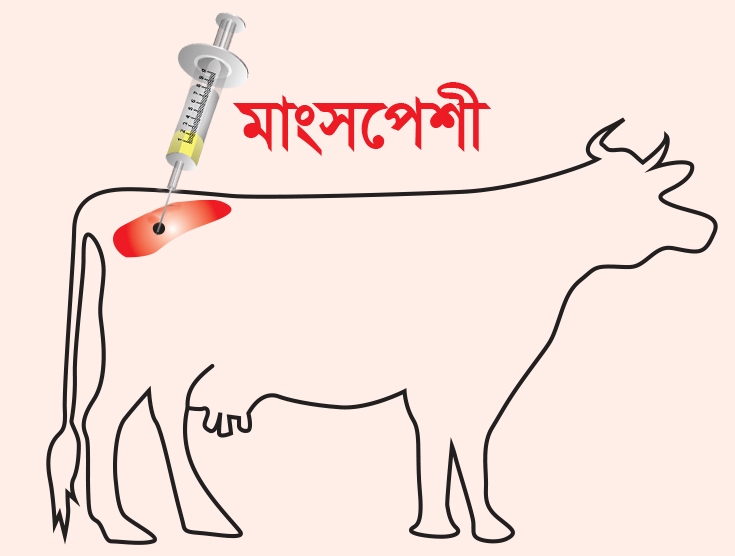
Dosage and Administration:
• Vaccine is recommended for cattle, goat, sheep and buffaloes as a prophylactic measure against Foot
and Mouth Disease
• For disease control by routine vaccination in endemic area
• For limiting the spread of the disease during outbreak
Side Effects:
Generally no significant side effects are noticed after intramuscular route vaccination. However, swellings at the injection site may occur after vaccination. It usually resolves within few days but may last few weeks in some cases. A slight increase in rectal temperature of up to 1.2 °C for 4 days may appear in some cases.
Precautions:
Infected and animals at the incubation period of disease, should not be vaccinated. The animals which are infected and suspected of being infected should not be vaccinated. Open vials should be used within 2 hours. Part-used vials of vaccine must be disposed.
Contraindications:
FMD vaccine is not recommended for the animals that are clinically sick or severely debilitated or under stress.
Use in Pregnancy and Lactation:
There is no contraindication of the vaccine for pregnant animals
Over Dosage:
Sufficient clinical data is not available.
Withdrawal Period:
Not required.
Storage:
The vaccine should be stored and transported between +2 0 C and +8 0 C. Antigenicity of the vaccine deteriorates if the temperature is allowed to rise above this range. Do not freeze the vaccine. Keep out of the reach of children.
Commercial Pack:
Khuravax Vet 5 Doses: Each vial contains 10 ml vaccine which is closed by chlorobutyl rubber stopper and flip off seal.
Khuravax Vet 10 Doses: Each vial contains 20 ml vaccine which is closed by chlorobutyl rubber stopper and flip off seal.